 |
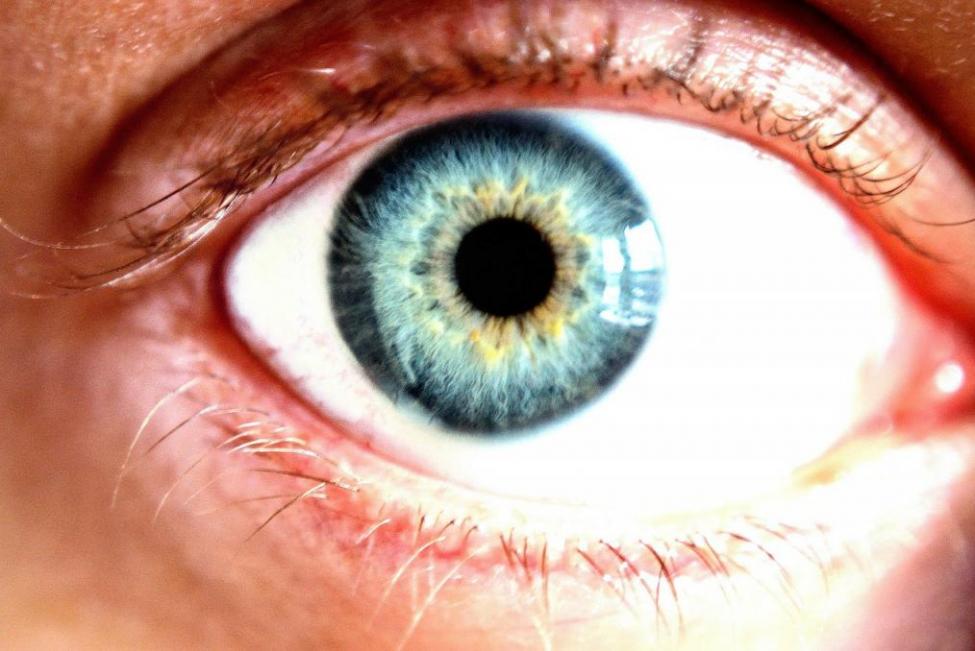
| This is a normal cornea, which is the clear layer that covers the colored portion of the front of the eye. The Food and Drug Administration approved a drug to treat neurotrophic keratitis in which the trigeminal nerve is damaged. |
By Allen Cone, UPI
The U.S. Food and Drug Administration approved the first medication to treat a rare eye disease affecting the cornea.
Dompe farmaceutici SpA, an Italian bio-pharmaceutical company, can market and sell the brand name drug Oxervate to treat neurotrophic keratitis in the United States, the FDA said in a release Wednesday. The drug was approved by the European Medicines Agency in 2017.
"While the prevalence of neurotrophic keratitis is low, the impact of this serious condition on an individual patient can be devastating," Dr. Wiley Chambers, an ophthalmologist in the FDA's Center for Drug Evaluation and Research, said in a press release. "In the past, it has often been necessary to turn to surgical interventions; these treatments are usually only palliative in this disease."
Neurotrophic keratitis has been estimated to afflict fewer than than five in 10,000 individuals, according to the FDA.
The degenerative disease affects the cornea, which is the clear layer that covers the colored portion of the front of the eye. With damage to the trigeminal nerve, there is decreased corneal sensitivity and poor corneal healing.
Other corneal eye diseases are keratoconus, which is the thinning and conical shape of the cornea, and Fuchs' dystrophy, which includes swelling of the cornea. The drug wasn't approved for these conditions.
Herpes simplex and herpes zoster viral infections are the most common causes, according to the American Academy of Ophthalmology.
In early stages, artificial tears are administered frequently and lubricant ointment at night. At stage 2, scleral contact lenses are used to assist in healing. Surgery is performed in a later stage.
Oxervate contains cenegermin-bkbj, a recombinant form of human nerve growth factor, which is a protein made by the human body.
"Neurotrophic keratitis can be disabling, hard to treat, and many patients do not respond well to existing therapies," said Reza Dana, a professor of ophthalmology at Harvard Medical School, said in a company press release. "By directly promoting corneal healing, Oxervate has the potential to change the way neurotrophic keratitis is treated, and may eventually result in a new standard of care for patients with this rare condition."
The drug underwent two clinical trials that involved 76 patients with neurotrophic keratitis over eight weeks. All eye drops -- including the studied medication, were administered six days a day.
In one study in Europe involving 52 participants, 72 percent of patients in the treatment group were completely healed. In a study in the United States that included 24 patients, 65.2 percent of treated patients were completely healed. The drug was compared with the use of artificial tears.
Oxervate was granted Priority Review designation -- an expedited application within six months of an application filing to treat serious conditions. Oxervate also received Orphan Drug designation, which provides incentives in the development of drugs for rare diseases.
Dompe was founded in 1940. Its Biotech Unit focuses specifically on the rare diseases field, ophthalmology, according to its website.
















COMMENTS